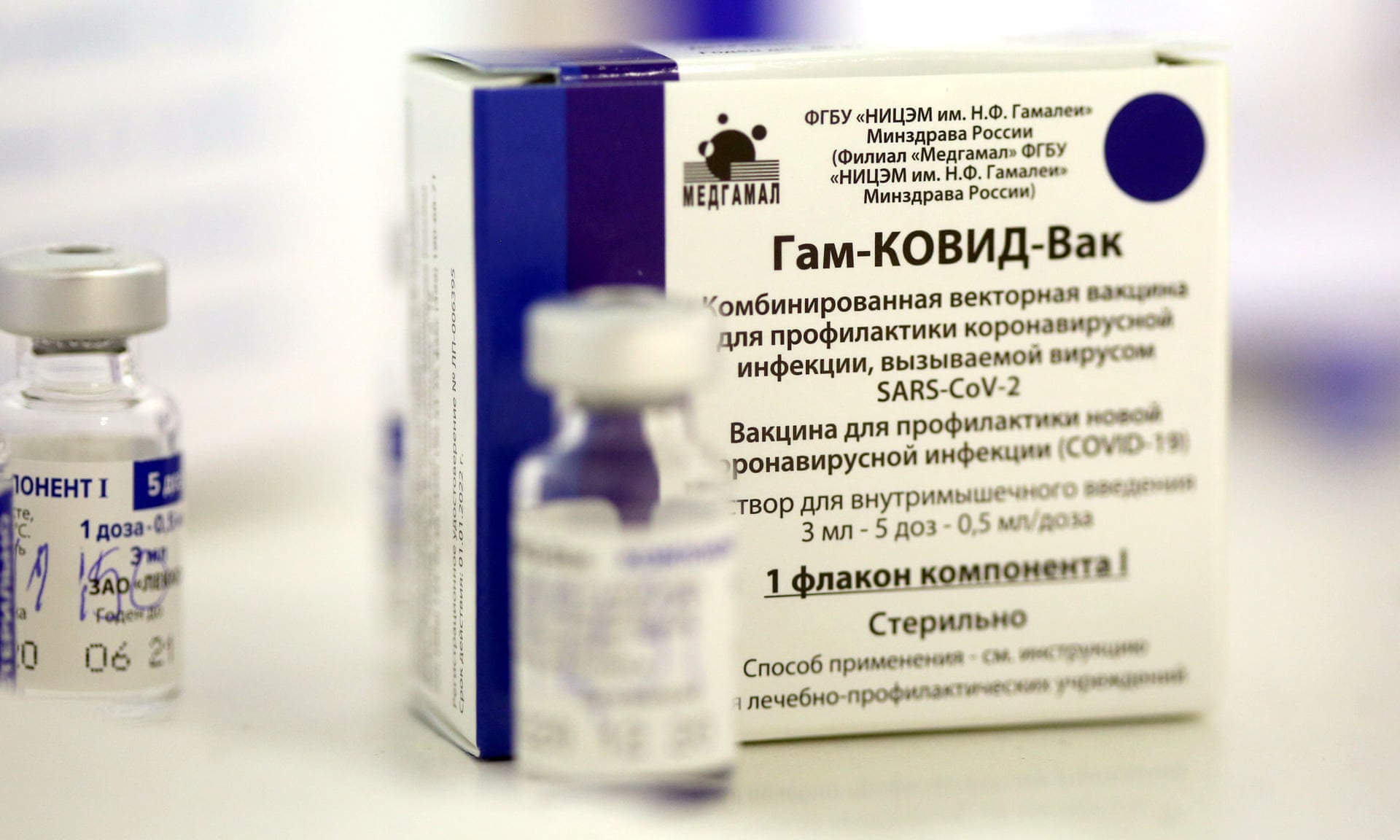
Russia's Sputnik V, which was initially criticized internationally for being rolled out before the final trial data had been released, has been ranked as the world's second Covid-19 vaccine in terms of regulatory approvals, surpassing the American-made Pfizer jab.
The ranking was based on publicly available data on vaccine registration, which demonstrated global demand for the first Russian-made coronavirus vaccine developed by the Moscow-based Gamaleya Research Institute of Epidemiology and Microbiology.
"SputnikV is now the world's second most popular CovidVaccine in terms of regulatory approvals," the Russian Direct Investment Fund (RDIF) tweeted on the Sputnik V official account on Friday.
Russia was the world's first country to register a coronavirus vaccine despite Sputnik V's incomplete clinical trials. Experts both at home and abroad warned to avoid its wider use before the studies are fully completed. However, the interest in the Russian-made vaccine has surged abroad after the prestigious Lancet medical journal published in early February the latest stage results that showed an impressive 92% efficacy for the vaccine. According to the results, Sputnik V is claimed to be safe and offers complete protection against hospitalization and death.
Sputnik V has already been registered under the emergency use authorization procedure without additional clinical trials in Algeria, Argentina, Bolivia, Serbia, Palestine, Paraguay and Venezuela. To date, the Sputnik V vaccine has been approved for use in 45 countries across the globe with an overall population of 1.2 billion people. However, Sputnik V has notably not yet been approved by Russia’s neighbor and regional rival, the EU.
Earlier, the European Medicines Agency (EMA) commenced a rolling review of the Russian Covid-19 vaccine Sputnik V to decide if the vaccine complies with efficacy, safety and quality standards in the EU. The rolling review will continue once enough information has been submitted to warrant official registration. Although EMA cannot predict the length of the review, it is not expected to take longer than the usual review.
According to the ranking, the AstraZeneca vaccine billed as AZD1222 topped the list of the most-approved Covid-19 vaccines in the world as it is registered in 49 countries. Co-invented by Oxford University and AstraZeneca, the AZD1222 vaccine, which was approved by European regulators but not by the Americans, showed 62% efficacy in preventing Covid-19 infections in its clinical trials. AstraZeneca joined hands with the Serum Institute of India to manufacture and ship the vaccine across the globe.
According to the vaccine developers, both the Oxford and the Sputnik V jabs use harmless viruses as a carrier to deliver a small fragment of the coronavirus to the body. While the Russian Sputnik V vaccine uses the unique technology of combining two different vectors based on human adenovirus, the Oxford-AstraZeneca's AZD1222 vaccine is based on a replication-deficient chimpanzee viral vector.
So far, the world's largest country has already registered three Covid-19 vaccines, including the Sputnik V shot, EpiVacCorona developed by the Siberia-based Vektor State Virology and Biotechnology Center, and CoviVac produced by the Moscow-based state-run Chumakov Centre. With the introduction of the third jab, Russia became the only country with three vaccines produced locally for the prevention of coronavirus infection.
The deadly virus, which was first identified in Wuhan, the capital of China’s Hubei province, in December, 2019, has exponentially spread around the globe. After cases of human-to-human transmissions were confirmed, the World Health Organization (WHO) declared the coronavirus outbreak an international public health emergency, but due to a sharp surge in cases, it was soon forced to make a new statement, declaring the outbreak a pandemic in 2020.
According to the latest data, the number of global coronavirus cases has reached the grim milestones of 117 million cases and about 2.6 million fatalities.






 President Aliyev emphasized the critical role of the North-South Transport Corridor in fostering transport cooperation between Azerbaijan and Russi...
President Aliyev emphasized the critical role of the North-South Transport Corridor in fostering transport cooperation between Azerbaijan and Russi...
 Russian Foreign Minister Sergei Lavrov has reasserted that Moscow has no intentions to stop the fighting in Ukraine, even if peace talks commence.
Russian Foreign Minister Sergei Lavrov has reasserted that Moscow has no intentions to stop the fighting in Ukraine, even if peace talks commence.
 Iran has refuted reports of alleged damage to Shimon Peres Negev Nuclear Research Centre located southeast of Dimona, Israel, during the recent air...
Iran has refuted reports of alleged damage to Shimon Peres Negev Nuclear Research Centre located southeast of Dimona, Israel, during the recent air...
 Iran and Pakistan have signed eight cooperation documents in various fields, and agreed to strengthen ties to fight terrorism in the region.
Iran and Pakistan have signed eight cooperation documents in various fields, and agreed to strengthen ties to fight terrorism in the region.



