The world's largest country is expected to register its second potential coronavirus vaccine this week following the completion of early-stage human trials.
The news comes as more than 100 Covid-19 vaccine candidates are under development around the globe, many of which are in human trial phases.
Addressing an international online conference titled "Pandemic 2020: Challenges, Solutions, Consequences," Russian Health Minister Mikhail Murashko announced Russia's plans to register a second vaccine that he says could bring a permanent end to the coronavirus pandemic.
"Today, two Russian vaccines are in the clinical trial (stage), while another one is registered," Russian news agency RIA Novosti quoted Murashko as saying on Tuesday during a conference at the Russian Presidential Academy of National Economy and Public Administration.
He also mentioned plans for one of the vaccines in clinical trials to be registered and moved to the production stage later this week.
Earlier, Russia’s consumer protection watchdog Rospotrebnadzor revealed that Russia’s second candidate Covid-19 vaccine, developed by the Siberia-based Vektor State Virology and Biotechnology Center, is expected to go through registration process by October 15.
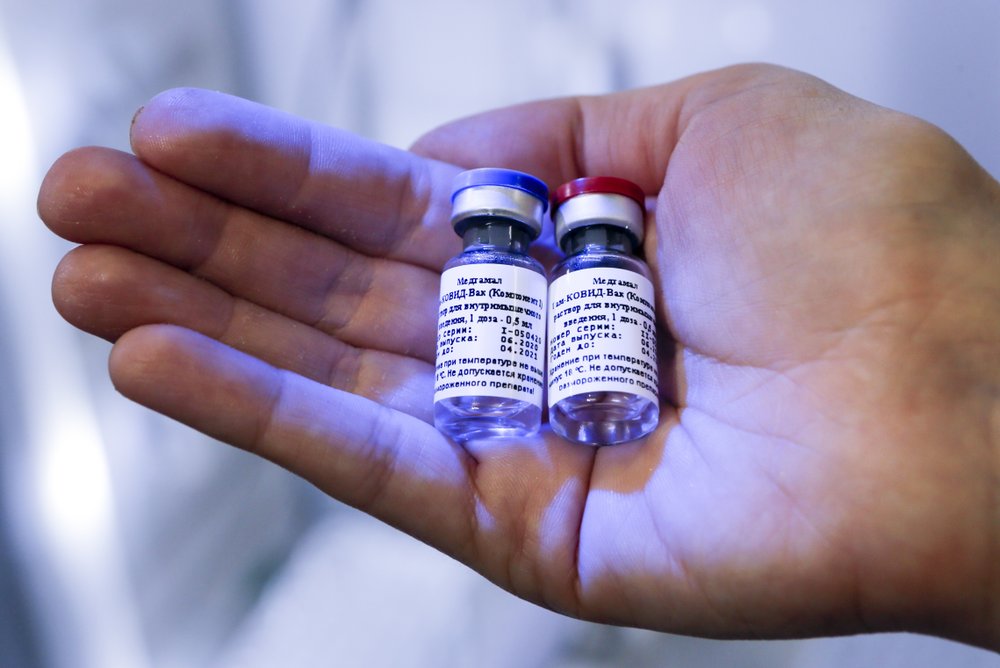
The new vaccine, called EpiVacCorona, would arrive less than 10 weeks after Russia became the first country in the world to approve a Covid-19 vaccine. In August, Russia reached a milestone in its fight against the coronavirus when Russian President Vladimir Putin announced the approval of a coronavirus vaccine for human use, declaring Russia's victory in the global vaccine race.
Developed by the Moscow-based Gamaleya Research Institute of Epidemiology and Microbiology under the Russian Health Ministry, Russia's first vaccine, named Sputnik-V, bears the same name as the world's first satellite launched in 1957 by the Soviet Union during the space race. The name signifies the country's success in being the first nation to have an approved vaccine.
However, the vaccine, first licensed by Russian authorities for use, has raised a wave of skepticism among experts from some western countries and scientists who began questioning its efficacy and safety, mainly because the vaccine had received approval before full clinical trials have been completed.
Amid the buzz to develop a potential vaccine against the novel coronavirus, human trials of the Russian Covid-19 vaccine are currently being tested on 40,000 volunteers in Moscow as part of a late-stage trial known as Phase 3. Additionally, the vaccine will be tested in the United Arab Emirates. The trials in the UAE are the second trials of the Sputnik V vaccine abroad, following the launch of trials in Belarus. Similar trials are also expected to begin in Venezuela in the near future.
Russia has fourth-highest number of confirmed Covid-19 infections worldwide, while the number of cases continues to rise. Russia confirmed a record 14,231 new daily Covid-19 cases on Wednesday, bringing the total number of confirmed cases in the country to 1,340,409. On the same day, Russia’s coronavirus crisis centre said 239 more deaths were confirmed in the last 24 hours, bringing the death toll to 23,205.
First identified in China, the novel coronavirus, or Covid-19, has infected more than 38.3 million people and killed more than one million worldwide, according to data compiled by Johns Hopkins University. Due to the fact that many people infected with the virus do not experience symptoms, the actual number of cases is likely to be significantly higher.






 Iran's senior military leaders described the drone and missile attack on Israel on April 14 night as “successful".
Iran's senior military leaders described the drone and missile attack on Israel on April 14 night as “successful".
 The number of evacuees from flooded areas in Kazakhstan has reached 97,852 people, including about 32,856 children since March 27.
The number of evacuees from flooded areas in Kazakhstan has reached 97,852 people, including about 32,856 children since March 27.
 Iranian President Ebrahim Raisi warned Israel that it would face a "real and extensive" response if it makes any "mistake" following Tehran’s missi...
Iranian President Ebrahim Raisi warned Israel that it would face a "real and extensive" response if it makes any "mistake" following Tehran’s missi...



